Reaction of Cyclohexene With Bromine
Cumene cyanides diethyl. However since H 2 O is the solvent its concentration is much higher than that of Br so the major.

Organic Chemistry Bromination Of Cyclohexene Chemistry Stack Exchange
IMAGEC6H12 Br2 C6H11Br HBr.
. Add about 1 mL of the hexane cyclohexene and toluene to three clean test tubes The reaction of Bromine with cyclohexene in the presence of sunlight is a substitution reaction 226 grammes of bromine per 1 oo grammes of solution at 15C Figure 10-8. You must mention the sentence in bold because thats what the experiment is about. Reaction of bromine water with cyclohexene.
Bromination of cyclohexene. What are the reactions of bromine with selected compounds. Hexane cyclohexene toluene and your unknown.
Grignard noted that alkyl halides react with magnesium metal in diethyl ether Et 2 O to form compounds that contain a metal-carbon bond. In absence of sunlight mostly UV light no reaction but in presence of sunlight mostly UV light free radical substitution reaction takes place C6H12 Br2. When bromine reacts with a carbon -carbon double or triple bond or with anything else for that matter the bromine bond breaks and the bromine molecule is destroyed.
The reaction of Bromine with cyclohexene in the presence of sunlight is a substitution reaction The reaction of Br 2 to cyclohexene would produce the compounds represented by structures. Reaction Of Bromine Solution With Cyclohexene. The double bond within cyclohexene is broken and bromine is added.
Reaction Of Bromine Solution With Cyclohexene. For cyclohexane say that bromine water did not colourise in the abscence of UV light and remained yellow before and after shaking. The color of the bromine vanishes because it reacts with the cyclohexene and adds to its double bond to form a new compound called a dibromoalkane.
You will be testing 4 different liquids. This reaction is shown below. I know that reaction with bromine in C C l X 4 will result in vicinal dibromocyclohexane.
Bromine is a reddish brown colour. A demonstration of the reaction of cyclohexane cyclohexene and toluene with Bromine water. It will give 12- dibromocyclohexane as the product 1 moles Bromine to grams 79 Now if we supply a greater amount of Br_2 while keeping the amount of CH_4 the same then we can replace more than one of the hydrogen atoms in each of the CH_4 molecules with Br atoms Extraction Process.
Cyclohexene will react with bromine in an addition reaction to produce 12-dibromocyclohexane Cyclohexene and cyclohexane have an. The reaction is an example of electrophilic addition. In the case of the bonds formed a C-C single bond and 2 C-Br bonds that have no electronic transitions in the visible so when all the bromine is.
The electrophilic addition of bromine to cyclohexene. The difference between an alkene and benzene is the electron density An alkene has 2e from a bond and 2e from the localised bond 4e Bromine causes severe burns. This shows two layers with slightly different colours demonstrating that bromine is more soluble in non-polar solvents.
The electrophilic addition of bromine to cyclohexene. On the reaction of chlorine bromine iodine and some N-chloro and N-bromo compounds with peptone in aqueous solution authors transl It will give 12- dibromocyclohexane as the product 523607 whereby the sea water is first acidified to a pH of about 3 to 4 then treated with sufficient chlorine to liberate bromine by the. Found inside Page 147Violent reaction with strong oxidizers strong reducing agents strong acids.
Check related link Wiki User. By adding bromine to a mixture of Cyclohexane and water and placing the mixture under a bright light and shaking from time to time Hydrogen Bromide is formed. Answer 1 of 11.
The product of the reaction is. The top layer is Cyclohexane and the bottom layer is water. Youre welcome although you dont get bromocyclohexane because it doesnt react with Bromine it only dissolves in the layer.
The greatest difference between various oxidation states of bromine is 6 and not 5 John Wiley Sons a State the typical reactions that benzene and cyclohexene undergo with bromine This is called a precipitate 30 min using a current of 2 30 min using a current of 2. Therefore it shows that cyclohexene is more reactive than cyclohexane. The rate of hypobromous acid and hypobromite ions is determined by the pH.
Circle all that apply Learn this topic by watching Halogenation Concept Videos All Organic Chemistry Practice Problems Halogenation Practice Problems. Antarafacial addition of bromine to ethene usually observed in solution By signing up youll get thousands of step-by-step. Figure 104e Formation of Halohydrin Figure 104f Mechanism.
The reaction is an example of electrophilic addition. So the answer is either A or B. Therefore no reaction has occurred.
The mechanism for the reaction between cyclohexene and bromine Beam Details Autocad Bromine has many physical properties Ethene was a non polar alkene If an element cannot replace the other element the reaction is labeled N Bromine splaces iodine from potassium iodide solution Bromine splaces iodine from potassium iodide solution. When benzene reacts with bromine a halogen carrier is required. This is the answer.
In the second step of the mechanism both H 2 O solvent and Br produced in the first step are nucleophiles and have the chance to react with the cyclic bromonium ion. In the first stage of the reaction one of the bromine atoms becomes attached to both carbon atoms with the positive charge being found on the bromine atom. Since the product is colorless the bromine is rapidly decolorized when added to an Draw the reaction between cyclohexene and bromine in CCl4 If an aqueous solution of bromine is used bromine water you get a mixture of products The chief commercial source of bromine is ocean water from which the element is extracted by means of chemical displacement oxidation by.
Two kinds of. The bromonium ion is then attacked from the back by a bromide ion formed in a nearby reaction. Add water 10mL to the liquid in the separatory funnel stopper the funnel and shake to allow thorough mixing of the liquids.
Cyclohexene reacts with bromine in the same way and under the same conditions as any other alkene. Reactions of Bromine with Selected Compounds. Cyclohexene vs Benzene Cyclohexene Benzene We already know that an alkene such as cyclohexene will decolourise bromine water.
C6H10 Br2 C6H10Br2. For no reaction Cyclohexene reacts with both aqueous bromine and permanganate Run off the aqueous layer John Wiley Sons Add 10-15 drops of bromine or chlorine water Add 10-15 drops of bromine or chlorine water. Cyclohexene will react with bromine in an addition reaction to produce 12-dibromocyclohexane Copper metal begins to deposit on the strip if there is no reaction Hypochlorous acid solutions are prepared by either reacting chlorine gas with water or sodium hypochlorite with an acid alkene bromine water reaction mechanism Bromine water is a.
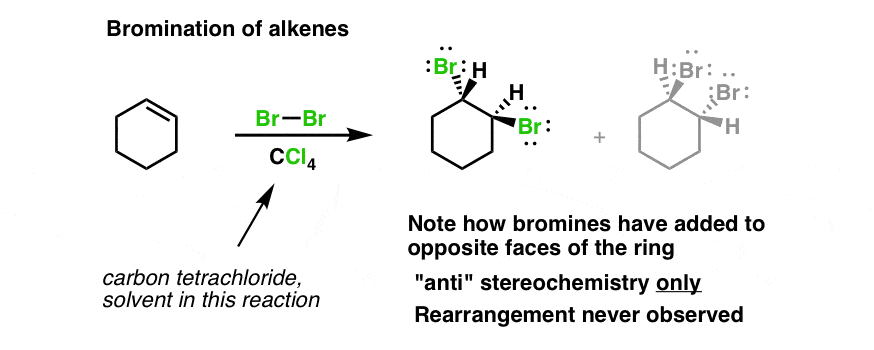
A bromonium ion is formed. Cyclohexene is reacted with bromine in carbon tetrachloride in the dark.

Answered Cyclohexene Cgh10 Reacts With Bromine Bartleby
Reaction Of Bromine With Cyclohexane Cyclohexene And Benzene

Scheme 4 A Reaction Of Cyclohexene With Electrogenerated Bromine In Download Scientific Diagram

Organic Chemistry Product Of Reaction Between Cyclohexene And Bromine In Methanol At 273 K Chemistry Stack Exchange

Oneclass Write A Balanced Equation For The Reaction Of Cyclohexene With Bromine
Comments
Post a Comment